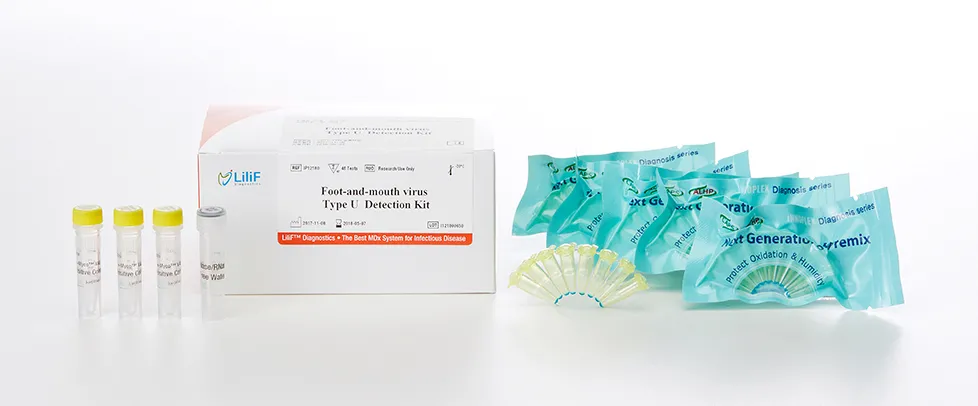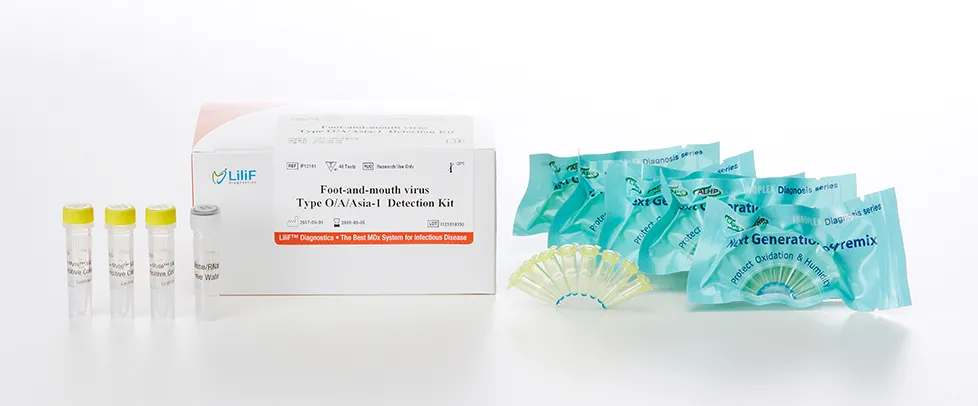
LiliF® FMDV type-O/A/Asia1 RT-PCR Kit
Background Information
The foot-and-mouth disease virus (FMDV) is the pathogen that causes foot-and-mouth disease. It is a picornavirus, the prototypical member of the genus Aphthovirus. The disease, which causes vesicles (blisters) in the mouth and feet of bovids, suids, ovids, caprids and other cloven-hoofed animals is highly infectious and a major plague of animal farming. The virus particle (25-30 nm) has an icosahedral capsid made of protein, without envelope, containing a single strand of ribonucleic acid (RNA) containing a positive encoding of its genome. When the virus comes in contact with the membrane of a host cell, it binds to a receptor site and triggers a folding-in of the membrane. Once the virus is inside the host cell, the capsid dissolves, and the RNA gets replicated, and translated into viral proteins by the cell's ribosomes using a cap-independent mechanism driven by the internal ribosome entry site element The synthesis of viral proteins include 2A 'cleavage' during translation. They include proteases that inhibit the synthesis of normal cell proteins, and other proteins that interact with different components of the host cell. The infected cell ends up producing large quantities of viral RNA and capsid proteins, which are assembled to form new viruses. After assembly, the host cell lyses (bursts) and releases the new viruses. The foot-and-mouth disease virus occurs in seven major serotypes: O, A, C, SAT-1, SAT-2, SAT-3, and Asia-1. These serotypes show some regionality, and the O serotype is most common.
LiliF™ FMDV type-O/A/Asia1 RT-PCR Kit is able to detect directly and specifically FMDV virus by CLP™ technology and Maxime ™ technology on the basis of a genetic database of target nucleic acid fragments. Therefore, this kit can diagnose very sensitive, fast and accurately. The kit contains a specific primer set for a highly conserved region based on current sequence alignments of FMDV virus, allowing the RNA detection. It can determine the infecting all serotype and accurately and sensitivity detect multiple detection genes at one time using the conventional RT-PCR method, and take only 3 hours for detection. Fast and sensitive detection of pathogen enables patients to get appropriate treatment and prevent the rapid spreading of disease by separating patients immediately. Each tube in the kit contains polymerase, reverse transcriptase, dNTPs, 10x reaction buffer, RNase inhibitor, specific primers for detection, and tracking dye for hot start PCR, except for the template-specific amplification of foot-and- It is very easy to add only distilled water and template for PCR.
Principle
• This product is an animal gene that is qualitatively detected by reverse transcription-polymerase chain reaction (RT-PCR) to detect foot-and-mouth disease virus type O, A, and Asia1 pathogens in animal tissues, saliva, Detection kit.
• In other words, DNase / RNase free water supplied from the viral RNA extracted from animal tissues, saliva, juice or blood can be rapidly and qualitatively detected simultaneously.
• This product is optimized for universal PCR machine with 0.2 ml PCR tube thermal block. Based on genetic database of foot and mouth disease virus, it is reacted by specific primer of foot and mouth virus type O, A, After completion of the reaction, make sure the target size band is detected using the electrophoresis unit and agarose gel.
Intended Use
• For Research Use Only, Not for use in diagnostic procedures.
• This kit is developed, designed, and sold for research purpose only. It is not intended to be used for human or animal diagnosis of diseases. Prior to using it for other purposes, the user must validate the system in compliance with the applicable law, directives, and regulations.
• This product is used for animals that are qualitatively detected by reverse transcription-polymerase chain reaction (RT-PCR) to detect foot-and-mouth disease virus type O, A, and Asia1 pathogens in animal tissues, saliva, Gene detection kit.
- Categories : PCR Kits, Diagnostic Use, Animal Diagnostics
.webp)
(1).webp)
(2).webp)
(3).webp)
(4).webp)
(5).webp)
(6).webp)
(7).webp)
(8).webp)
(9).webp)
(10).webp)
(11).webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)