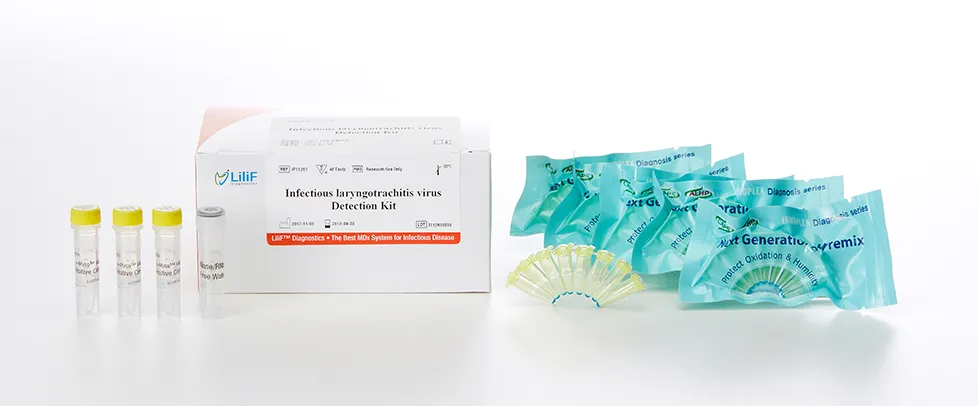
LiliF® ILTV PCR Kit
Background Information
Infectious laryngotracheitis virus (ILTV) causes a serious, worldwide-occurring respiratory disease of chickens which affects growth and egg production and may lead to death of the animals. The acute phase of infection lasts between 1 and 2 weeks and is often associated with clinical signs like gasping, coughing, expectoration of bloody mucus, and conjunctivitis. Subsequently, an asymptomatic latent infection of the central nervous system can be established. Gallid herpesvirus 1 (GaHV-1) (also known as Avian herpesvirus 1) is a virus of the family Herpesviridae that causes avian infectious laryngotracheitis. It was originally recognized as a disease of chickens in the United States in 1926. The disease also occurs in pheasants. The disease is usually referred to as infectious laryngotracheitis or simply LT in the poultry industry. It is widely viewed as one of the most contagious viruses that affect the poultry industry. A confirmed case will usually result in the establishment of a quarantine zone around the farm. Inside this quarantine zone, poultry workers will avoid poultry farms to prevent the spread of the virus. GaHV-1 is shed in respiratory secretions and transmitted by droplet inhalation or via fomites. A previously unexposed flock will develop cases for two to eight weeks following introduction. The incubation period is two to eight days.
LiliF™ ILTV PCR Kit is able to detect directly and specifically ILTV by CLP™ technology and Maxime™ technology on the basis of a genetic database of target nucleic acid fragments. Therefore, this kit can diagnose very sensitive, fast and accurately. The kit contains a specific primer set for a highly conserved region based on current sequence alignments of glycoprotein gene of ILTV, allowing the DNA detection. It can determine the infecting all serotype and accurately and sensitivity detect multiple detection genes at one time using the conventional PCR method, and take only 2 hours for detection. Fast and sensitive detection of pathogen enables patients to get appropriate treatment and prevent the rapid spreading of disease by separating patients immediately.
Principle
• This product is a qualitative PCR testing product with CLP™ technology which provided flexibility in Tm (melting temperature) of primer design for optimization of reaction condition, and maximizes PCR specificity and sensitivity through the control of non-specific priming.
• The assay is a PCR that discriminates ILTV in one reaction. The assay is composed of two principal steps: (1) nucleic acid extraction from specimens, and (2) amplification of the target extracted nucleic acid fragment using specific primers pair.
Intended Use
• For Research Use Only, Not for use in diagnostic procedures.
• This kit is developed, designed, and sold for research purpose only. It is not intended to be used for human or animal diagnosis of diseases. Prior to using it for other purposes, the user must validate the system in compliance with the applicable law, directives, and regulations.
• This product is research reagent of infectious disease for professional use to restrict the public use for animal diseases.
- Categories : PCR Kits, Diagnostic Use, Animal Diagnostics
.webp)
(1).webp)
(2).webp)
(3).webp)
(4).webp)
(5).webp)
(6).webp)
(7).webp)
(8).webp)
(9).webp)
(10).webp)
(11).webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)