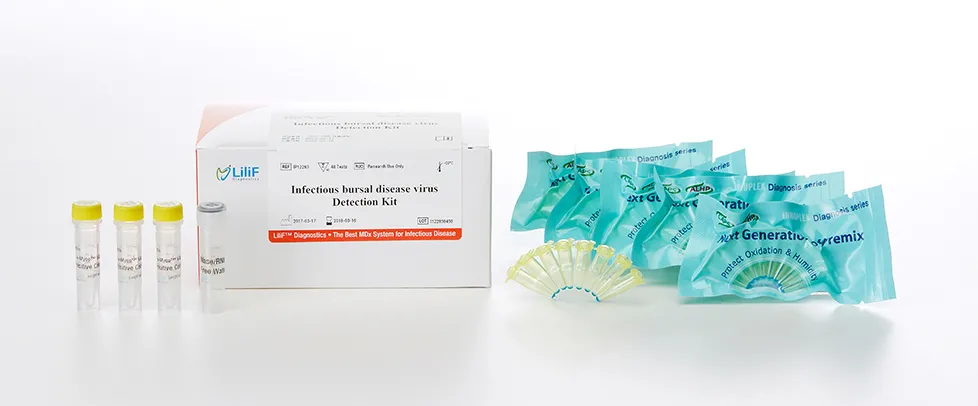
LiliF® IBDV Real-time RT-PCR Kit
Background Information
Infectious bursal disease, IBD (also known as Gumboro disease, infectious bursitis and infectious avian nephrosis) is a highly contagious disease of young chickens caused by infectious bursal disease virus (IBDV), characterized by immunosuppression and mortality generally at 3 to 6 weeks of age. The disease was first discovered in Gumboro, Delaware in 1962. It is economically important to the poultry industry worldwide due to increased susceptibility to other diseases and negative interference with effective vaccination. In recent years, very virulent strains of IBDV (vvIBDV), causing severe mortality in chicken, have emerged in Europe, Latin America, South-East Asia, Africa and the Middle East. Infection is via the oro-fecal route, with affected bird excreting high levels of the virus for approximately 2 weeks after infection. IBDV is a double stranded RNA virus that has a bi-segmented genome and belongs to the genus Avibirnavirus of family Birnaviridae.
There are two distinct serotypes of the virus, but only serotype 1 viruses cause disease in poultry. At least six antigenic subtypes of IBDV serotype 1 have been identified by in vitro cross-neutralization assay. Viruses belonging to one of these antigenic subtypes are commonly known as variants, which were reported to break through high levels of maternal antibodies in commercial flocks, causing up to 60 to 100 percent mortality rates in chickens. With the advent of highly sensitive molecular techniques, such as reverse transcription polymerase chain reaction (RT-PCR) and restriction fragment length polymorphism (RFLP), it became possible to detect the vvIBDV, to differentiate IBDV strains, and to use such information in studying the molecular epidemiology of the virus.
LiliF™ IBDV Real-time RT-PCR Kit is able to detect directly and specifically IBDV by real-time PCR technology on the basis of a genetic database of target nucleic acid fragments. Therefore, this kit can diagnose very sensitive, fast and accurately. The kit contains a specific primer and probe set for a highly conserved region based on current sequence alignments of all type of IBDV, allowing the RNA detection. It can determine the infecting all serotype and accurately and sensitivity detect multiple detection genes at one time using the real-time RT-PCR (quantitative) method, and take only 2 hours for detection. Fast and sensitive detection of pathogen enables patients to get appropriate treatment and prevent the rapid spreading of disease by separating patients immediately.
Principle
• This product is a qualitative Real-time PCR testing product with 5’ nuclease assay technology and CLP™ technology which provided flexibility in Tm (melting temperature) of primer design for optimization of reaction condition, and maximizes PCR specificity and sensitivity through the control of non-specific priming.
• The assay is a real-time RT-PCR that discriminates IBDV in one reaction. The assay is composed of two principal steps: (1) nucleic acid extraction from specimens, and (2) amplification of the target extracted nucleic acid fragment using fluorescent probe and specific primers pair.
• The internal positive control (IPC) has been introduced to individual tube of the kit to verify the successful amplification reaction. The IPC is co-amplified with target band from clinical specimens.
Intended Use
• For Research Use Only, Not for use in diagnostic procedures.
• This kit is developed, designed, and sold for research purpose only. It is not intended to be used for human or animal diagnosis of diseases. Prior to using it for other purposes, the user must validate the system in compliance with the applicable law, directives, and regulations.
• This product is research reagent of infectious disease for professional use to restrict the public use for animal diseases.
- Categories : PCR Kits, Diagnostic Use, Animal Diagnostics
.webp)
(1).webp)
(2).webp)
(3).webp)
(4).webp)
(5).webp)
(6).webp)
(7).webp)
(8).webp)
(9).webp)
(10).webp)
(11).webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)