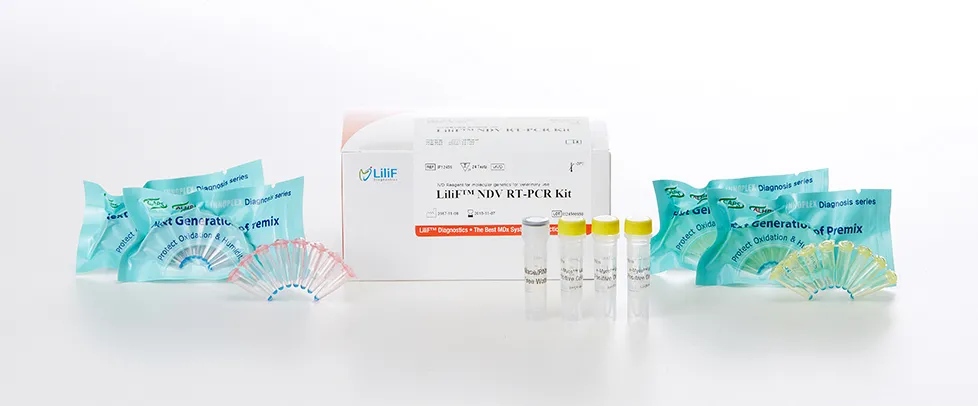
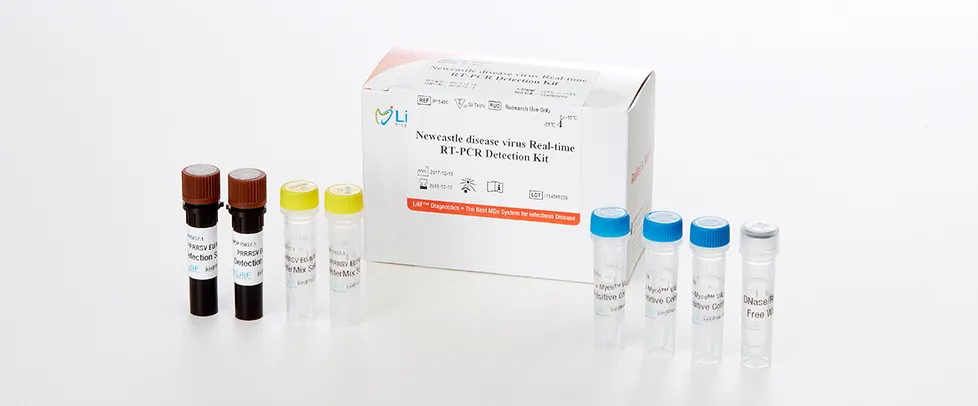
LiliF® NDV RT-PCR Kit
Background Information
Newcastle disease is a contagious viral bird disease affecting many domestic and wild avian species; it is transmissible to humans. It was first identified in Java, Indonesia, in 1926, and in 1927, in Newcastle-upon-Tyne, England (whence it got its name). However, it may have been prevalent as early as 1898, when a disease wiped out all the domestic fowl in northwest Scotland. Its effects are most notable in domestic poultry due to their high susceptibility and the potential for severe impacts of an epizootic on the poultry industries. It is endemic to many countries. Exposure of humans to infected birds (for example in poultry processing plants) can cause mild conjunctivitis and influenza-like symptoms, but the Newcastle disease virus (NDV) otherwise poses no hazard to human health. Interest in the use of NDV as an anticancer agent has arisen f rom the ability of NDV to selectively kill human tumour cells with limited toxicity to normal cells. No treatment for NDV exists, but the use of prophylactic vaccines and sanitary measures reduces the likelihood of outbreaks. Signs of infection with NDV vary greatly depending on factors such as the strain of virus and the health, age and species of the host. The incubation period for the disease ranges f rom two to 15 days. An infected bird may exhibit several signs, including respiratory signs (gasping, coughing), nervous signs (depression, inappetence, muscular tremors, drooping wings, twisting of head and neck, circling, complete paralysis), swelling of the tissues around the eyes and neck, greenish, watery diarrhea, misshapen, rough- or thin-shelled eggs and reduced egg production. In acute cases, the death is very sudden, and, in the beginning of the outbreak, the remaining birds do not seem to be sick. In flocks with good immunity, however, the signs (respiratory and digestive) are mild and progressive, and are followed after seven days by nervous symptoms, especially twisted heads.
LiliF™ NDV RT-PCR Kit is able to detect directly and specifically Newcastle disease virus by CLP™ technology and Maxime™ technology on the basis of a genetic database of target nucleic acid fragments. Therefore, this kit can diagnose very sensitive, fast and accurately. The kit contains a specific primer set for a highly conserved region based on current sequence alignments of Newcastle disease virus, allowing the RNA detection. It can determine the infecting all serotype and accurately and sensitivity detect multiple detection genes at one time using the conventional RT-PCR method, and take only 2 hours for detection. Fast and sensitive detection of pathogen enables patients to get appropriate treatment and prevent the rapid spreading of disease by separating patients immediately.
Principle
• This product is a qualitative RT-PCR testing product with 5’ nuclease assay technology and CLP™ technology which provided flexibility in Tm (melting temperature) of primer design for optimization of reaction condition, and maximizes PCR specificity and sensitivity through the control of non-specific priming.
• The assay is a conventional RT-PCR that discriminates non pathogenic NDV and pathogenic NDV in one reaction. The assay is composed of two principal steps:
(1) nucleic acid extraction f rom specimens, and (2) amplification of the target extracted nucleic acid fragment using fluorescent probe and specific primers pair.
Intended Use
• For in vitro diagnostics, IVD Reagents for molecular genetics for zoonosis disease.
• Permission No. 133-007 of Medical Devices for veterinary use authorized by Animal and Plant Quarantine Agency of Korea
• This kit is developed, designed, and sold for IVD purpose.
• This product is research reagent of infectious disease for professional use to restrict the public use for animal diseases.
- Categories : PCR Kits, Diagnostic Use, Animal Diagnostics
.webp)
(1).webp)
(2).webp)
(3).webp)
(4).webp)
(5).webp)
(6).webp)
(7).webp)
(8).webp)
(9).webp)
(10).webp)
(11).webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)
.webp)